Label G-Actin with Atto488 and Cy5 for in vitro Polymerization
Mainly ref to Yujie Sun 08/25/2009 notebook and Pollard Biophysical J., 88(2) 1387-1402, 2005
Get F-actin from Xiangdong Li's Lab, Institute of Zoology, Chinese Academy of Science.
First of all, depolymerize the F-actin for 3 days and centrifuge at 100,000 xg for 2hrs to obtain activated dark G-actin.
Secondly, remove the DTT and reduce the -S-S- on G-actin by 100 fold molar excess of TCEP and polymerize the G-actin to F-actin at RT for 30min by adding ATP and high concentration salt ion. Then label the F-actin with 10-fold molar excess Atto488-Maleimide and Cy5-Maleimide at a pH level not more than 7.5 overnight on a vertical rotor at 4°C room.
Thirdly, separate the labeled F-actin out of G-actin and free dye by centrifuge at 100,000 xg for 2hrs. Then, depolymerize the labeled F-actin and centrifuge to obtain activated dye labeled G-actin.
Fourthly, polymerize and centrifuge to obtain the activated dye labeled F-actin.
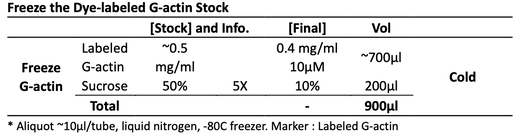
Lastly, dialyze and depolymerize for 2 days and centrifuge to obtain the final activated can-polymerized dye-labeled G-actin. Aliquot and freeze the labeled G-actin.
Get F-actin from Xiangdong Li's Lab, Institute of Zoology, Chinese Academy of Science.
First of all, depolymerize the F-actin for 3 days and centrifuge at 100,000 xg for 2hrs to obtain activated dark G-actin.
Secondly, remove the DTT and reduce the -S-S- on G-actin by 100 fold molar excess of TCEP and polymerize the G-actin to F-actin at RT for 30min by adding ATP and high concentration salt ion. Then label the F-actin with 10-fold molar excess Atto488-Maleimide and Cy5-Maleimide at a pH level not more than 7.5 overnight on a vertical rotor at 4°C room.
Thirdly, separate the labeled F-actin out of G-actin and free dye by centrifuge at 100,000 xg for 2hrs. Then, depolymerize the labeled F-actin and centrifuge to obtain activated dye labeled G-actin.
Fourthly, polymerize and centrifuge to obtain the activated dye labeled F-actin.
Lastly, dialyze and depolymerize for 2 days and centrifuge to obtain the final activated can-polymerized dye-labeled G-actin. Aliquot and freeze the labeled G-actin.
Prepare the Dialyzing Bags
Cut the dialyzing bags to 100~200mm-long pieces. Boil the dialyzing bags in 1L 2% (w/v) NaHCO3 and 1mM EDTA·Na2 pH 8.0 for 10 min to remove the oil and metal ion . Rinse the dialyzing bags with plenty of ddH2O. Then re-boil the dialyzing bags in 1L 1mM EDTA·Na2 pH 8.0 for 10 min to remove the metal ion on the bags. Rinse the dialyzing bags with plenty of ddH2O.
Store the bags in ddH2O for a long term in 4C freezer. Keep moist.
Dialyze the F-actin to get G-actin
Day 0 - Sat Feb 23 night - Wen Feb 27 2013 morning
314μM (12.5mg/ml, ~800μl) F-Actin in Tris/HCl buffer (2mM Tris/HCl, 40mM KCl, without DTT and ATP) from Xiangdong Li, Institute of Zoology, Chinese Academy of Science.
Note : the actin solution is pretty sticky. Wash the tube with Buffer G.
Dilute the F-actin with Buffer G with DTT to 6mg/ml, finally there's ~1,600μl solution.
Dialyze the F-Actin in Buffer G with DTT in 4C room for three days against three changes of Buffer G with DTT (pre-freeze the buffer!!!) to get activated G-actin.
Day 1 - Wed, Feb 27 2013
Collect ~1,600μl F-actin & G-actin solution in EP tube from the dialyze bag. Transfer to high speed centrifuge tube. To separate the F-actin from the G-actin, centrifuge at 100,000 xg for 2 h at 4C (Beckman, super-high speed centrifuge) in Dongyi Xu's Lab, 6F, Life Science, PKU.
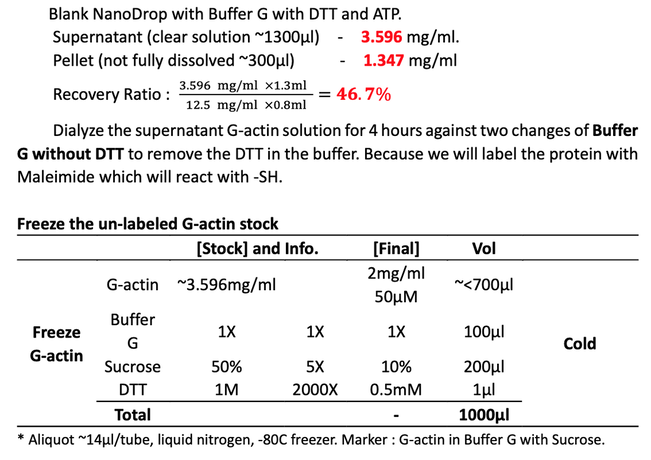
Take the crude G-actin in the top 80% of the supernatant (~1,300μl). Re-suspend the pellet with the rest ~300μl buffer. Measure the concentration of unlabeled actin by NanoDrop, BIOPIC.
Cut the dialyzing bags to 100~200mm-long pieces. Boil the dialyzing bags in 1L 2% (w/v) NaHCO3 and 1mM EDTA·Na2 pH 8.0 for 10 min to remove the oil and metal ion . Rinse the dialyzing bags with plenty of ddH2O. Then re-boil the dialyzing bags in 1L 1mM EDTA·Na2 pH 8.0 for 10 min to remove the metal ion on the bags. Rinse the dialyzing bags with plenty of ddH2O.
Store the bags in ddH2O for a long term in 4C freezer. Keep moist.
Dialyze the F-actin to get G-actin
Day 0 - Sat Feb 23 night - Wen Feb 27 2013 morning
314μM (12.5mg/ml, ~800μl) F-Actin in Tris/HCl buffer (2mM Tris/HCl, 40mM KCl, without DTT and ATP) from Xiangdong Li, Institute of Zoology, Chinese Academy of Science.
Note : the actin solution is pretty sticky. Wash the tube with Buffer G.
Dilute the F-actin with Buffer G with DTT to 6mg/ml, finally there's ~1,600μl solution.
Dialyze the F-Actin in Buffer G with DTT in 4C room for three days against three changes of Buffer G with DTT (pre-freeze the buffer!!!) to get activated G-actin.
Day 1 - Wed, Feb 27 2013
Collect ~1,600μl F-actin & G-actin solution in EP tube from the dialyze bag. Transfer to high speed centrifuge tube. To separate the F-actin from the G-actin, centrifuge at 100,000 xg for 2 h at 4C (Beckman, super-high speed centrifuge) in Dongyi Xu's Lab, 6F, Life Science, PKU.
Take the crude G-actin in the top 80% of the supernatant (~1,300μl). Re-suspend the pellet with the rest ~300μl buffer. Measure the concentration of unlabeled actin by NanoDrop, BIOPIC.
Label G-actin with Maleimide Modified Dye
Day 1 - Wed, Feb 27 2013
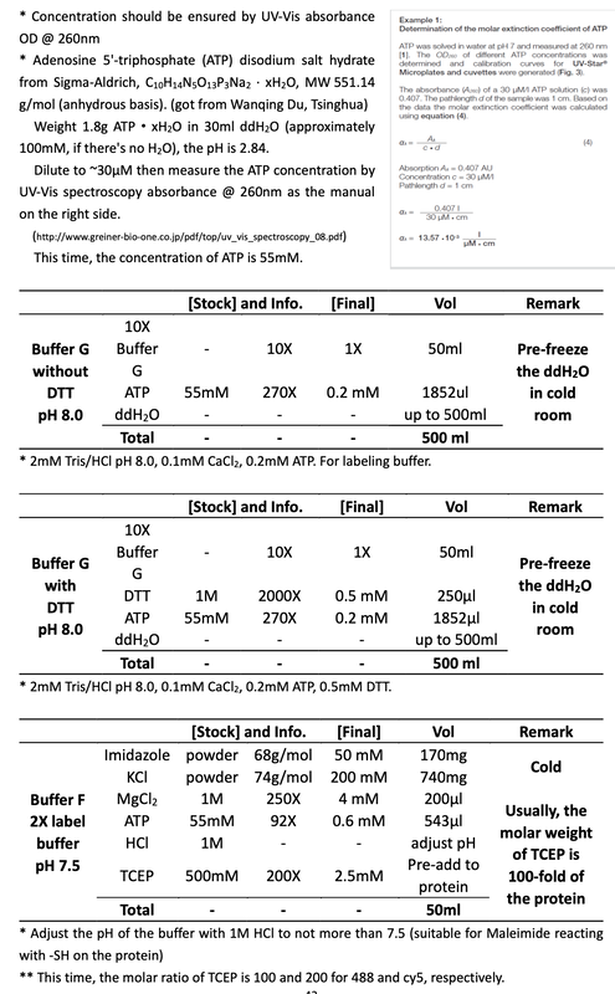
1~2 hours before the labeling, dilute 278μl 3.596 mg/ml G-actin(1mg protein) to 2mg/ml with 222μl cold Buffer G with TCEP (2mM Tris/HCl, pH 8.0, 0.2mM ATP, 0.1mM CaCl2, 100-fold molar excess TCEP as the protein (for 1mg/ml actin equal 25μM, so the TCEP concentration should be 2.5mM)) to reduce and protect the -SH on the actin protein side.
Note : This time, the molar ratio of TCEP is 100 and 200 for 488 and cy5, respectively.
Mix 500μl 2mg/ml actin with 500μl 2X Buffer F (50mM imidazol, 200mM KCl, 4mM MgCL2, 0.6mM ATP, 2.5mM TCEP), (final concentration 1mg/ml), polymerize ~30min at RT to get F-actin.
1ml 1mg/ml (25μM, 42kDa) F-actin filaments [25,000 μM·μl]
+ 0.25mg dye (~1000g/mol) in 50μl DMF [250,000 μM·μl]
For Atto488-Maleimide, use 200μl DMF dilute 1 tube(1mg/tube) dye, then take 50μl;
For Cy5-Maleimide, take 5 tubes (0.05mg/tube, 10μl/tube) aliquots.
Dropwise add the dye solution to the F-actin solution while stirring, and vertically stir the solution gently overnight at 4C room on a vertical rotor. Minimize bubble formation.
Day 2 - Thur, Feb 28 2013
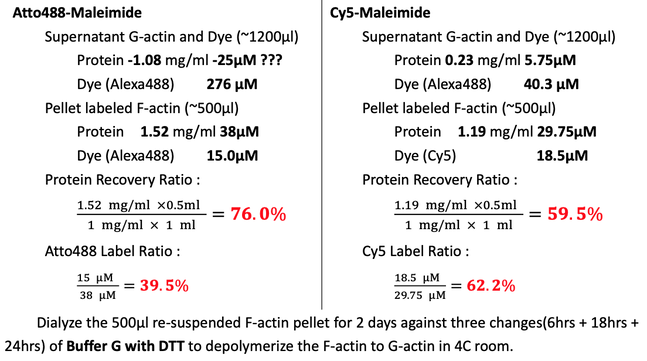
Clarify the labeled F-actin at 500 xg for 5min. Transfer the supernatant, ~1000μl yellow solution for Atto488 and blue for Cy5 to centrifuge tubes. Wash the labeling EP tube twice with 100μl Buffer G with DTT. Centrifuge at 100,000 xg for 2hrs to pellet the labeled F-actin filaments at 4C (Beckman, super-high speed centrifuge) in Dongyi Xu's Lab, 6F, Life Science, PKU.
Collect the ~1200μl supernatant G-actin. Actively re-suspend and shear the F-actin pellet in 500μl (200μl + 300μl) Buffer G with DTT. Minimize bubble formation.
Briefly sonicate the 500μl re-suspended labeled F-actin pellet in water bath (50% power) for 1 min to fully dissolve the labeled F-actin. Optionally, shear again to fully dissolve the F-actin.
Note : the pellet should be dark stained of the dye color.
Measure the protein concentration by NanoDrop, BIOPIC. Blank with Buffer G with DTT.
Day 1 - Wed, Feb 27 2013
1~2 hours before the labeling, dilute 278μl 3.596 mg/ml G-actin(1mg protein) to 2mg/ml with 222μl cold Buffer G with TCEP (2mM Tris/HCl, pH 8.0, 0.2mM ATP, 0.1mM CaCl2, 100-fold molar excess TCEP as the protein (for 1mg/ml actin equal 25μM, so the TCEP concentration should be 2.5mM)) to reduce and protect the -SH on the actin protein side.
Note : This time, the molar ratio of TCEP is 100 and 200 for 488 and cy5, respectively.
Mix 500μl 2mg/ml actin with 500μl 2X Buffer F (50mM imidazol, 200mM KCl, 4mM MgCL2, 0.6mM ATP, 2.5mM TCEP), (final concentration 1mg/ml), polymerize ~30min at RT to get F-actin.
1ml 1mg/ml (25μM, 42kDa) F-actin filaments [25,000 μM·μl]
+ 0.25mg dye (~1000g/mol) in 50μl DMF [250,000 μM·μl]
For Atto488-Maleimide, use 200μl DMF dilute 1 tube(1mg/tube) dye, then take 50μl;
For Cy5-Maleimide, take 5 tubes (0.05mg/tube, 10μl/tube) aliquots.
Dropwise add the dye solution to the F-actin solution while stirring, and vertically stir the solution gently overnight at 4C room on a vertical rotor. Minimize bubble formation.
Day 2 - Thur, Feb 28 2013
Clarify the labeled F-actin at 500 xg for 5min. Transfer the supernatant, ~1000μl yellow solution for Atto488 and blue for Cy5 to centrifuge tubes. Wash the labeling EP tube twice with 100μl Buffer G with DTT. Centrifuge at 100,000 xg for 2hrs to pellet the labeled F-actin filaments at 4C (Beckman, super-high speed centrifuge) in Dongyi Xu's Lab, 6F, Life Science, PKU.
Collect the ~1200μl supernatant G-actin. Actively re-suspend and shear the F-actin pellet in 500μl (200μl + 300μl) Buffer G with DTT. Minimize bubble formation.
Briefly sonicate the 500μl re-suspended labeled F-actin pellet in water bath (50% power) for 1 min to fully dissolve the labeled F-actin. Optionally, shear again to fully dissolve the F-actin.
Note : the pellet should be dark stained of the dye color.
Measure the protein concentration by NanoDrop, BIOPIC. Blank with Buffer G with DTT.
Day 3 - Fri, Mar 01 2013
Continue the dialysis. Change dialyzing buffer.
Day 4 - Sat Mar 02 2013
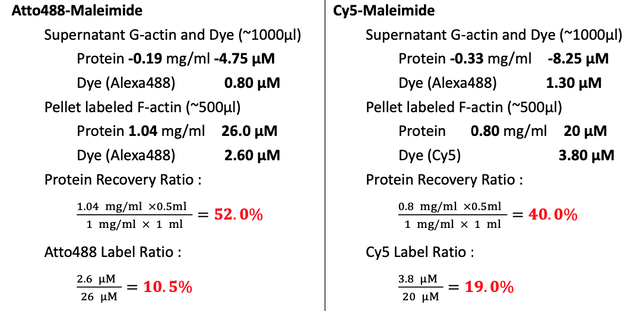
Collect ~600μl depolymerized and labeled G-actin. Wash the dialyzing bag with 200μl Buffer G with DTT. Transfer the ~800μl solution to centrifuge tube. Centrifuge at 100,000 xg for 2hrs to pellet the F-actin filaments at 4C (Beckman, super-high speed centrifuge) in Dongyi Xu's Lab, 6F, Life Science, PKU. Note : there should not be much pellet in the centrifuge tube.
Measure the protein concentration by NanoDrop, BIOPIC. Blank with Buffer G with DTT.
Continue the dialysis. Change dialyzing buffer.
Day 4 - Sat Mar 02 2013
Collect ~600μl depolymerized and labeled G-actin. Wash the dialyzing bag with 200μl Buffer G with DTT. Transfer the ~800μl solution to centrifuge tube. Centrifuge at 100,000 xg for 2hrs to pellet the F-actin filaments at 4C (Beckman, super-high speed centrifuge) in Dongyi Xu's Lab, 6F, Life Science, PKU. Note : there should not be much pellet in the centrifuge tube.
Measure the protein concentration by NanoDrop, BIOPIC. Blank with Buffer G with DTT.
Day 5 - Sun Mar 03 2013
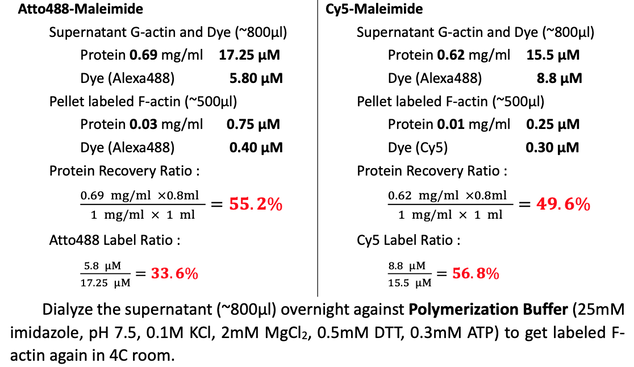
Collect ~800μl activated labeled F-actin. Wash the dialyzing bag with 200μl Polymerization Buffer. Transfer the ~1000μl solution to centrifuge tube. Centrifuge at 100,000 xg for 2hrs to pellet the labeled F-actin filaments at 4C (Beckman, super-high speed centrifuge) in Dongyi Xu's Lab, 6F, Life Science, PKU.
Note : there shoμld be a lot of labeled pellet at the bottom of the centrifuge tube.
Actively re-suspend and shear the pellet with 500μl Buffer G with DTT.
Measure the protein concentration by NanoDrop, BIOPIC. Blank with Polymerization Buffer and Buffer G with DTT for supernatant and pellet, respectively.
Collect ~800μl activated labeled F-actin. Wash the dialyzing bag with 200μl Polymerization Buffer. Transfer the ~1000μl solution to centrifuge tube. Centrifuge at 100,000 xg for 2hrs to pellet the labeled F-actin filaments at 4C (Beckman, super-high speed centrifuge) in Dongyi Xu's Lab, 6F, Life Science, PKU.
Note : there shoμld be a lot of labeled pellet at the bottom of the centrifuge tube.
Actively re-suspend and shear the pellet with 500μl Buffer G with DTT.
Measure the protein concentration by NanoDrop, BIOPIC. Blank with Polymerization Buffer and Buffer G with DTT for supernatant and pellet, respectively.
Note : If blank NanoDrop with Buffer G with DTT, the measured protein concentration in the Polymerization Buffer is 0.59 mg/ml because of the higher concentration of ATP.
Dialyze the re-suspended F-actin pellet for 2 days against three changes(6hrs + 18hrs + 24hrs) of Buffer G with DTT to depolymerize the F-actin to G-actin in 4C room.
Day 6 - Mon Mar 04 2013
Continue the dialysis. Change the dialyzing buffer.
Day 7 - Tue Mar 05 2013
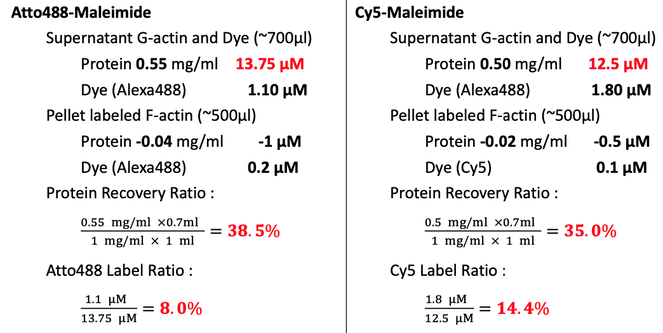
Collect ~500μl depolymerized and labeled G-actin. Wash the dialyzing bag with 200μl Buffer G with DTT. Transfer the ~700μl solution to centrifuge tube. Centrifuge at 100,000 xg for 2hrs to pellet the inactivated labeled F-actin filaments at 4C (Beckman, super-high speed centrifuge) in Dongyi Xu's Lab, 6F, Life Science, PKU.
Note : there should not be much pellet in the centrifuge tube.
Collect the supernatant (~700μl) and re-suspend the pellet in 500μl Buffer G with DTT.
Measure the protein concentration by NanoDrop, BIOPIC. Blank with Buffer G with DTT.
Dialyze the re-suspended F-actin pellet for 2 days against three changes(6hrs + 18hrs + 24hrs) of Buffer G with DTT to depolymerize the F-actin to G-actin in 4C room.
Day 6 - Mon Mar 04 2013
Continue the dialysis. Change the dialyzing buffer.
Day 7 - Tue Mar 05 2013
Collect ~500μl depolymerized and labeled G-actin. Wash the dialyzing bag with 200μl Buffer G with DTT. Transfer the ~700μl solution to centrifuge tube. Centrifuge at 100,000 xg for 2hrs to pellet the inactivated labeled F-actin filaments at 4C (Beckman, super-high speed centrifuge) in Dongyi Xu's Lab, 6F, Life Science, PKU.
Note : there should not be much pellet in the centrifuge tube.
Collect the supernatant (~700μl) and re-suspend the pellet in 500μl Buffer G with DTT.
Measure the protein concentration by NanoDrop, BIOPIC. Blank with Buffer G with DTT.
Note : This time, the molar ratio of TCEP to actin is 100X and 200X for Atto488 and Cy5 sample, respectively. It proves that TCEP could open and protect -SH very well.
Prepare Phalloidin Solution
Phalloidin from Amanita Phalloides, P2141-1mg, Sigma-Aldrich. MW : 788.87g/mol.
Note : Phalloidin is very toxic. Swallowing, touching or breathing is very dangerous.
Dissolve 1mg Phalloidin in 1.27ml DMSO to get a 1mM Pahlloidin Stock (1000X) and aliquot them to 20μl in EP tube at RT.
Observe the labeled actin
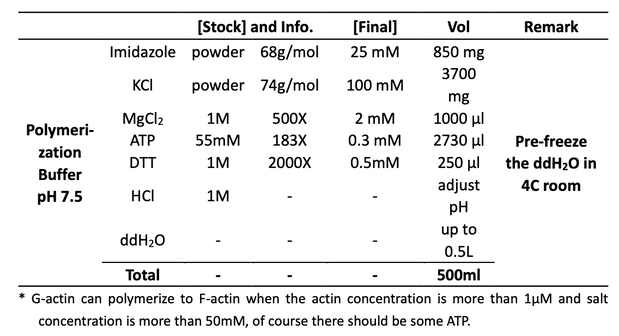
18μl Polymerization Buffer (25mM imidazole, pH 7.5, 0.1M KCl, 2mM MgCl2, 0.5mM DTT, 0.3mM ATP, this time the polymerization buffer is from Mar 02 polymerization) + 2μl 10% ~10μM dye-labeled actin + 0.2μl 1mM Phalloidin to stable the actin
Note : the final concentration of Phalloidin should be 1μM, but cannot pippet 0.02μl.
Note : Cut tip or blend by tip, avoid any shear force.
Protect from light! Polymerize the labeled G-actin to F-actin at RT for ~10min while assembling a normal four-channeled flow chamber by double side tape between a slide and a cleaned coverslip.
Flow the actin in channel and observe the actin on microscope.
Prepare Phalloidin Solution
Phalloidin from Amanita Phalloides, P2141-1mg, Sigma-Aldrich. MW : 788.87g/mol.
Note : Phalloidin is very toxic. Swallowing, touching or breathing is very dangerous.
Dissolve 1mg Phalloidin in 1.27ml DMSO to get a 1mM Pahlloidin Stock (1000X) and aliquot them to 20μl in EP tube at RT.
Observe the labeled actin
18μl Polymerization Buffer (25mM imidazole, pH 7.5, 0.1M KCl, 2mM MgCl2, 0.5mM DTT, 0.3mM ATP, this time the polymerization buffer is from Mar 02 polymerization) + 2μl 10% ~10μM dye-labeled actin + 0.2μl 1mM Phalloidin to stable the actin
Note : the final concentration of Phalloidin should be 1μM, but cannot pippet 0.02μl.
Note : Cut tip or blend by tip, avoid any shear force.
Protect from light! Polymerize the labeled G-actin to F-actin at RT for ~10min while assembling a normal four-channeled flow chamber by double side tape between a slide and a cleaned coverslip.
Flow the actin in channel and observe the actin on microscope.
Experience & Conclusion
For Cy5 : 59.5% 49.6% 40.0% 35.0%
- The concentration of ATP should be measured and calculated carefully using UV-Vis Spectroscope or NanoDrop @260nm absorbance.
- About 10L Buffer G with DTT had been used for dialysis for 2 color actin.
- For this kind of acitn-like precious protein, it's better to use low-binding EP tubes and pippet tips. Rinse tubes, bags and tips with Buffer after transfer to reduce the loss.
- TCEP can protect the -SH very well. Before the labeling of -SH with -maleimide, add ~200 fold molar excess of TCEP in the protein solution to get a higher labeling efficiency. Labeling efficiency are 8.0%and 14.4% for Atto488 and Cy5, respectively.
- After several times dialysis and centrifuge, the protein recovery ratio is
For Cy5 : 59.5% 49.6% 40.0% 35.0%
- Be extremely careful when dissolve the Phalloidin.
- Coverslip and Polymerization Buffer are clean enough for imaging.
- Atto488 and Cy5 labeled actin are in good condition for in vitro polymerization experiment.
- Cy5 actin will photobleach without GLOX or Trolox